- Research
- Open access
- Published:
Characterization of YABBY transcription factors in Osmanthus fragrans and functional analysis of OfYABBY12 in floral scent formation and leaf morphology
BMC Plant Biology volume 24, Article number: 589 (2024)
Abstract
Background
The plant-specific YABBY transcription factor family plays important roles in plant growth and development, particularly leaf growth, floral organ formation, and secondary metabolite synthesis.
Results
Here, we identified a total of 13 OfYABBY genes from the Osmanthus fragrans genome. These 13 OfYABBY genes were divided into five subfamilies through phylogenetic analysis, and genes in the same subfamily showed similar gene structures and conserved protein motifs. Gene duplication promoted the expansion of the OfYABBY family in O. fragrans. Tissue-specific expression analysis showed that the OfYABBY family was mainly expressed in O. fragrans leaves and floral organs. To better understand the role of OfYABBY genes in plant growth and development, OfYABBY12 was selected for heterologous stable overexpression in tobacco, and OfYABBY12-overexpressing tobacco leaves released significantly fewer volatile organic compounds than wild-type tobacco leaves. Overexpression of OfYABBY12 led to the downregulation of NtCCD1/4 and decreased β-ionone biosynthesis. Correspondingly, a dual-luciferase assay showed that OfYABBY12 negatively regulated the expression of OfCCD4, which promotes β-ionone synthesis. Furthermore, tobacco leaves overexpressing OfYABBY12 were curled and wrinkled and had significantly reduced leaf thickness and leaf inclusions and significantly extended flower pistils (styles).
Conclusion
Overall, the results suggest that the OfYABBY gene family may influence the biosynthesis of the floral scent (especially β-ionone) in O. fragrans and may regulate leaf morphogenesis and lateral organs.
Introduction
Osmanthus fragrans is an important ornamental evergreen tree or shrub that is widely cultivated for its pleasing floral scent. It is commonly used in the landscaping and fragrance industries, where it has significant ornamental and economic value [1, 2]. Many O. fragrans cultivars have been cultivated in China for more than 2,500 years. These cultivars have significant differences in floral scent, floral color, leaf shape, and leaf color [3,4,5]. Previous studies have shown that the YABBY gene family not only participates in the formation and development of plant leaves and floral organs but also affects the biosynthesis of secondary metabolites, such as terpene floral metabolites [6,7,8]. In this study, it was hypothesized that the phenotypic difference in the leaves, flowers, and floral scents of different O. fragrans cultivars may be regulated by the O. fragrans YABBY family.
The YABBY family is a class of plant-specific transcription factors [9]. Due to the roles these transcription factors play in many of the biological processes of plants, the YABBY family has attracted great interest from researchers. The YABBY proteins contain two conserved domains, an N-terminal C2C2 zinc finger domain and a C-terminal YABBY domain. In Arabidopsis thaliana, Solanum lycopersicum, and other dicotyledonous plants, the YABBY transcription factors are classified into five subfamilies, namely CRABS CLAW (CRC), FILAMENTOUS FLOWER (FIL)/YABBY3 (YAB3), INNER NO OUTER (INO), YABBY2 (YAB2), and YABBY5 (YAB5). However, in Oryza sativa, Zea mays, and other monocotyledons, contain only four subfamilies and lack the YAB5 subfamily [9]. It has been indicated that the YABBY transcription factors in monocotyledonous and dicotyledonous plants have undergone functional divergence, and four gene duplication events have occurred in the YABBY family, leading to genes with innovative or redundant functions [10].
Some studies have shown that YABBY transcription factors are related to abaxial axis cell differentiation in lateral organs, thereby affecting leaf growth [11, 12] or the development of floral organs [13, 14] and fruit (seeds) [15, 16]. The YABBY family also participates in the biosynthesis of primary and secondary plant metabolites [8, 17, 18] and in response to biotic and abiotic stress [19,20,21]. In A. thaliana, AtYAB2, AtFIL, AtYAB3, and AtYAB5 are expressed in the cotyledons, leaves, petals, stamens, and carpels and participate in regulating bud and leaf development and the formation of leaf polarity and floral organs [6, 7]. However, AtCRC is only expressed in nectaries and carpels, regulating the abaxial development of these structures [22]. Similarly, AtINO is only expressed in the abaxial axis of the outer integument and mainly regulates ovule development, thereby affecting seed growth and development [23, 24]. In S. lycopersicum, SlYABBY1 regulates leaf and flower sizes [25], SlYABBY2 regulates pericarp development and fruit maturation [26], and SlCRCa regulates flower and fruit size [27]. In Chrysanthemum morifolium, CmDRP (FlL/YAB3 subfamily) can regulate gibberellin biosynthesis to affect chrysanthemum plant height [28]. In addition, one study has shown that YABBY genes have dual functions, as they can act as both an activator and a repressor of secondary metabolites [29]. For example, MsYABBY5 of Mentha spicata negatively regulates the synthesis of monoterpenes [8], whereas in Artemisia annua, AaYABBY5 positively regulates the biosynthesis of artemisinin [17] and flavonoids [18]. Hence, YABBY transcription factors show tissue specificity in plants and play a variety of roles in plant growth and development.
Here, a comprehensive analysis of the O. fragrans YABBY gene family was conducted based on whole genome data, and OfYABBY12 was stably overexpressed in tobacco to investigate whether OfYABBY12 affects the growth and development of leaves and floral organs, as well as the synthesis of volatile organic compounds (VOCs). These results help to understand the OfYABBY family in O. fragrans and provide insight into the function of the OfYABBY12 gene. The findings have important significance for further study of the functions of the OfYABBY family in O. fragrans.
Materials and methods
Plant materials
The experimental material was three-year-old O. fragrans ‘Rixiang Gui’ cuttings obtained from our O. fragrans germplasm resource bank (Jiangsu, China). Various tissues, including roots, stems, young leaves (from current-year branchlets), and mature leaves (from non-current-year branchlets) were sampled on March 26, 2022, and flowers at the full blooming stage were sampled on October 9, 2022. Nicotiana benthamiana and Nicotiana tabacum ‘K326’ seeds were saved by our group and planted in the greenhouse of Nanjing Forestry University under the following growth conditions: a 16/8 h light/dark cycle, a light intensity of 110 µmol photons m− 2 s− 1 white light, a temperature of 25 ± 2 °C, and 60–70% relative humidity.
Identification of O. fragrans YABBY transcription factors and protein physicochemical analysis
The whole genome sequence of O. fragrans was obtained from the O. fragrans ‘Rixiang Gui’ genomic database [30]. HMMER software (v3.0) was then used to screen for family members in the Pfam YABBY (PF04690) domain [31]. Three online tools, Batch CD-search (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi), Pfam (http://pfam.xfam.org/search#searchBatchBlock), and SMART (http://smart.embl.de/smart/batch.pl), were used to validate the YABBY domains. In addition, ExPASy (https://web.expasy.org/compute_pi/) was employed to predict the molecular weight and isoelectric point of the identified YABBY protein sequences.
Gene structure, conserved motif, and cis-acting element analysis
In this study, DNAMAN software (v6.0.40) was used for multi-sequence alignment and the analysis of the conserved protein domains of YABBY amino acid sequences in O. fragrans. In addition, MEME online software (http://meme-suite.org/) was used to analyze the conserved motifs of the OfYABBY protein sequences. The gene sequences 2,000 bp upstream of the start codon were obtained from the O. fragrans genome sequences, and the plantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) was used to retrieve the obtained sequences to analyze the potential cis-acting elements in the promoter. Finally, TBtools software (v1.120) [32] was used to visualize the gene structure, conserved motifs, and cis-acting elements of OfYABBYs.
Phylogenetic, chromosome localization, and gene duplication analysis
Genomic data were obtained from different databases, including A. thaliana (https://www.arabidopsis.org/), S. lycopersicum (https://solgenomics.net/), O. sativa (http://rice.uga.edu/), and Z. mays (https://maize-pangenome.gramene.org/). Furthermore, the protein sequences of YABBYs from Vitis vinifera [15], Camellia sinensis [33], Mimulus lewisii [34], M. spicata [8], Chimonanthus praecox [35], and A. annua [17] were obtained from relevant references.
Clustal X2.1 was used for the sequence alignment of the YABBY protein sequences of O. fragrans and other plants. In addition, MEGA X software was utilized to construct phylogenetic trees using the maximum likelihood method (Bootstrap = 1000) [36], and Fig Tree software (v1.4.3) was employed for visualization.
The chromosome locations of the OfYABBY genes were obtained based on the O. fragrans genome GFF3 file, and TBtools was used for visualization. Multiple Collinearity Scan Toolkits (MCScanX) and Ka/Ks calculators (NG) were used to further analyze the gene duplication events of the OfYABBY genes.
RNA extraction and quantitative reverse-transcription polymerase chain reaction (qRT-PCR) analyses
The RNAprep Pure Plant Kit (Tiangen, Beijing, China) was used to obtain the total RNA of plant materials, and One-Step gDNA Removal and cDNA Synthesis Super Mix (Transgen, Beijing, China) were used for reverse transcription to obtain complementary DNA (cDNA).
Primer Premier 5.0 software was used to design the qRT-PCR primers (Table S1). The ABI StepOne Plus PCR system and SYBR® Premix Ex Taq™ II Kit (Takara, Dalian, China) were used to measure gene expression levels. In addition, 10-fold diluted cDNA was used as a template, and OfACTIN was used as an internal reference gene [37]. The 2−ΔΔCT method [38] was employed to calculate the relative expression level. Biological triplicates were set up for each qRT-PCR reaction, and technical triplicates were set up for each biological triplicate.
Subcellular localization and transactivation assay
The cDNAs of OfYABBY3, OfYABBY5, OfYABBY7, OfYABBY8, OfYABBY12, and OfYABBY13 were cloned (primers shown in Table S2) and inserted into pCAMBIA1300 vectors to build the pCAMBIA1300-GFP-OfYABBY constructs. These constructed plasmids were transformed into N. benthamiana leaves by Agrobacterium tumefaciens strain GV3101 (Weidi Biotechnology, Shanghai, China). After 48 h, a Zeiss LSM 710 confocal microscope (Zeiss, Oberkochen, Germany) was used to observe fluorescence signals.
The cDNAs of OfYABBY3, OfYABBY5, OfYABBY7, OfYABBY8, OfYABBY12, and OfYABBY13 were cloned into the pGBKT7 vector (primers shown in Table S2). These recombination vectors and the pGBKT7 empty vector were transformed into Saccharomyces cerevisiae (AH109). The suspended yeast was then diluted to different concentrations, and 5 µL dilutions were plated on SD-Trp, SD-Trp/Ade, and SD-Trp/Ade (X-α-gal) culture media. These were cultured at 30 °C in the dark for 3 d, and the growth status was then observed.
Transformation of OfYABBY12 in N. tabacum
A. tumefaciens cells carrying the pCAMBIA1300-OfYABBY12 plasmid were cultured overnight, and the culture products were collected when the OD600 reached 0.4–0.5. A solution of 10 mM MgCl2, 10 mM MES, and 150 mM acetosyringone was used to resuspend the Agrobacterium cultures for transformation. In addition, tobacco (N. tabacum ‘K326’) seeds were sterilized and sown on MS medium, and when grown to three to four leaves, sterile tobacco leaves were cut into 0.5 cm × 0.5 cm cubes (that is, explants) and infiltrated with Agrobacterium cultures for 10 min. Subsequently, after 3 d of co-cultivation in the dark on the co-culture medium (MS + 2.25 mg/L 6-BA + 0.3 mg/L NAA), the explants were transferred to selective medium (MS + 2.25 mg/L 6-BA + 0.3 mg/L NAA + 400 mg/L cefotaxime + 100 mg/L kanamycin). The culture was kept at 25 °C with a 16/8 h light/dark cycle with the medium changed every 15 d. When the callus and resistant shoots emerged, the explants were transferred to germination medium (MS + 0.1 mg/L 6-BA + 0.01 mg/L NAA + 400 mg/L cefotaxime + 100 mg/L kanamycin). When the shoots grew to around 2 cm in length, the explants were transferred to rooting medium (MS + 400 mg/L cefotaxime + 100 mg/L kanamycin). Finally, after the roots were developed and mature, the plantlets were removed from the medium and planted in sterile soil.
After one month of growth, a Plant Rapid Genome Extraction kit (Tsingke, Wuhan, China) was used for a positive test of pCAMBIA1300-OfYABBY12 transgenic and wild-type (WT) tobacco leaves. Positive plants and WT plants were used for semi-quantitative validation, and transgenic lines with high levels of expression were selected for phenotype observation, scanning electron microscopy, gas chromatography–mass spectrometry (GC–MS), and RNA sequencing (RNA-Seq).
GC–MS volatile analysis
Headspace solid-phase microextraction (HS-SPME) was used to collect VOCs released from tobacco leaves or flowers. The samples of fresh leaves or flowers were ground in liquid nitrogen, 1 g of leaf or flower powder was placed in a 10-mL SPME vial with 3 mL NaCl saturated solution, and 1 µL of 5000-fold diluted ethyl caprate was added as the internal standard. Each vial was placed at room temperature (25 ± 2 °C) for 30 min and then extracted at 55 °C for 30 min using an extraction head composed of 65 µM polydimethylsiloxane (PDMS)/divinylbenzene (DVB) fiber (Supelco Co., Bellefonte, PA, USA) for GC–MS detection. GC–MS sequencing was conducted according to a previously described protocol [30].
Scanning electron microscopy
Fresh tobacco leaf samples were fixed in formalin–acetic acid–ethyl alcohol (FAA) at 4 °C for 48 h. The samples were then dehydrated sequentially in a gradient ethanol series, and after dehydration, the samples were vacuum-dried and sprayed with a gold film layer. Finally, the samples were placed under an environmental scanning electron microscope (Quanta 200, FEI Inc, Netherlands) for observation and photographs.
RNA-Seq analysis
Total RNA was extracted from mature WT and OfYABBY12-overexpressing (OfYABBY12-OE) tobacco leaves using an RNAprep Pure Plant Kit (Tiangen, Beijing, China). Each sample had three biological replicates. RNA-Seq was performed by the Guangzhou Gene Denovo Biotechnology Co. (Guangzhou, China) using the Illumina HiSeq2500 platform. All sequencing data were uploaded to the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) database (https://www.ncbi.nlm.nih.gov/sra/) under the accession number SRP450701.
Dual-luciferase transient expression assay
The coding sequence (CDS) of OfYABBY12 was amplified via PCR using gene-specific primers (Table S2) and connected to the pGreenII 62-SK vector to generate an effector, which was transformed into the A. tumefaciens strain GV3101 (pSoup). Then, the A. tumefaciens cells carrying the effector and reporter (pGreenII 0800-LUC-OfCCD4, preserved by our group) plasmids were mixed in a 4:3 ratio (v/v) and injected into N. benthamiana leaves. After 48 h, the luciferase (LUC) signal was detected using BERTHOLD NightSHADE LB 985 (Berthold, Bad Wildbad, Germany), and LUC and renilla luciferase (REN) were detected using a Dual-Luciferase Reporter Assay System kit (Yeasen Biotechnology, Shanghai, China).
Statistical analysis
One-way analysis of variance (ANOVA), Duncan’s multiple range test, and Student’s t-test were performed using IBM SPSS Statistics (version 20.0). A p-value smaller than 0.05 was considered significant. A minimum of three biological replicates were utilized for all experiments. Bar graphs were created using Origin Pro 2018. Heat maps were generated using TBtools software (version 1.120). Principal component analysis (PCA) and orthogonal partial least squares discrimination analysis (OPLS-DA) of VOCs were performed using SIMCA-13.0 software (version 13.0.3).
Results
Identification and classification of YABBY gene family members in O. fragrans
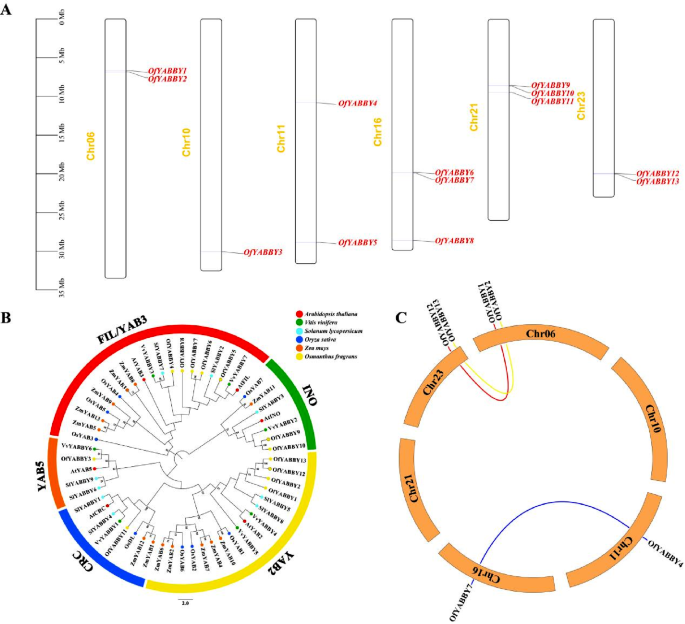
In this study, HMMER was used to identify 19 candidate YABBY genes in the O. fragrans whole genome database, and six sequences without intact structural domains were removed using NCBI-CDD, Pfam, and SMART. A total of 13 YABBY genes were identified in the O. fragrans genome. The OfYABBY genes were named based on their position on chromosomes as OfYABBY1–OfYABBY13, and these 13 genes were located on O. fragrans chromosomes 6, 10, 11, 16, 21, and 23 (Fig. 1A). The length of OfYABBY proteins ranged from 149 aa (OfYABBY13) to 247 aa (OfYABBY2), and the protein molecular weight ranged from 16.69 kDa (OfbYABBY13) to 27.39 kDa (OfbYABBY2). The theoretical isoelectric point (pI) was 6.18–9.3, and OfYABBY5, OfYABBY8, OfYABBY9, and OfYABBY10 were acidic proteins (pI < 7.00), while the remaining proteins were basic (Table S3).
The sequence alignment results of the 13 OfYABBY proteins revealed that all OfYABBY proteins contained two highly conserved protein domains, of which one was a C2C2 zinc finger domain in the N terminal, and one was a YABBY domain in the C terminal (Fig. S1A). In addition, the 13 OfYABBY genes contained four to seven introns, of which most OfYABBY genes contained six introns. OfYABBY5 and OfYABBY8 contained the greatest number of introns at seven. Overall, OfYABBY genes that clustered together shared identical or similar exon/intron structures. The conserved motifs of the OfYABBY family were analyzed (Table S4). All members contained at least two motifs, motifs 1 and 2, and genes that clustered together had similar motifs (Fig. S1B). These genes may have similar functions.
Interactions between cis- and trans-acting factors can regulate gene expression. The promoters and their upstream cis-acting elements were analyzed to predict their corresponding gene functions. In the O. fragrans YABBY gene family, every gene promoter contained four to 10 cis-acting elements (Fig. S2). Most were considered involved in light response, including GT1-motif, Box 4, TCT-motif, G-Box, AE-box, GA-motif, ATCT-motif, GATA-motif, chs-CMA1a, 3-AF1 binding, I-box, AE-box, TCCC-motif, MRE, and ACE. The responses to gibberellin, abscisic acid, salicylic acid, auxin, and methyl jasmonate included TATC-box, P-box, GARE-motif, ABRE, TCA-element, TGA-box, TGA-element, TGACG-motif, and CGTCA-motif. LTR, MBS, ARE, and TC-rich repeats might be involved in responding to drought, anaerobic, and low temperature stress. In addition, cis-acting elements such as CAT-box and HD-Zip 1 were involved in the developmental regulation of plant tissues and organs. This means that OfYABBYs may be involved in the responses to many plant hormones and environmental stress and play a role in plant growth and development.
Phylogenetic analysis and gene duplication
To understand the phylogenetic relationship between various YABBYs, 56 YABBY proteins from dicotyledonous plants (O. fragrans, A. thaliana, S. lycopersicum, and V. vinifera [15]) and monocotyledonous plants (O. sativa and Z. mays) were used to construct a phylogenetic tree (Fig. 1B). Based on the classification of A. thaliana YABBY proteins [39], the 13 OfYABBYs were divided into five subfamilies, namely FIL/YAB3, YAB5, YAB2, CRC, and INO. The FIL/YAB3 subfamily included five OfYABBY proteins, and the YAB2 subfamily included four OfYABBY proteins. In addition, the INO subfamily included two YABBY proteins, and the CRC and YAB5 subfamilies each included one OfYABBY (Table S3). As predicted, phylogenetic analysis showed that OfYABBYs had the closest phylogenetic relationship to YABBYs from dicotyledonous plants. The YABBYs of dicotyledonous plants were all clustered together. Moreover, the YABBYs of monocotyledonous plants, such as rice (O. sativa), formed a parallel branch, consistent with previous reports [9]. This shows that YABBY genes may function in the differentiation of monocotyledonous and dicotyledonous plants and that they are conserved in dicotyledonous plants.
In addition, phylogenetic analysis of O. fragrans YABBY protein sequences was conducted based on YABBYs in M. lewisii, C. sinensis, C. praecox, A. annua, and M. spicata. This is because their functions have been sufficiently discussed, such as MlYAB1/2/3/5, CsFILa/b, and CsYAB2, which are involved in leaf development regulation [33, 34], and CpFIL, CpCRC, CpYABBY2, CpYABBY5-1/-2, AaYABBY5 and MsYABBY5, which are involved in secondary metabolite biosynthesis [8, 17, 18, 35]. Based on the results (Fig. S3), it was deduced that the functions of OfYABBY1/2/3/4/5/6/7/8/12/13 genes of the FIL/YAB3, YAB2, and YAB5 subfamilies in O. fragrans may be related to leaf development or secondary metabolite synthesis. In addition, as shown in Fig. 1B, the FIL/YAB3 and YAB2 subfamilies contained more OfYABBY genes than other subfamilies, exhibiting significant gene duplication. However, because the number of O. fragrans YABBY family members was low, gene collinearity analysis did not find tandem repeat OfYABBY genes, but three segmental duplication of OfYABBY genes (OfYABBY1/OfYABBY12, OfYABBY2/OfYABBY13, and OfYABBY4/OfYABBY7) were detected (Fig. 1C).
Chromosomal distribution, phylogenetic relationships, and collinearity analysis of Osmanthus fragrans YABBY genes. (A) Chromosome locations of the OfYABBY genes. (B) Phylogenetic analysis of YABBYs from O. fragrans, Arabidopsis thaliana, Vitis vinifera, Solanum lycopersicum, Oryza sativa and Zea mays. Numbers at the nodes indicate bootstrap values; values lower than 50% are not shown. The sequences of the YABBYs used for phylogenetic relationship analysis are listed in Table S5. (C) Collinearity analysis for all OfYABBY genes
Expression patterns of OfYABBYs
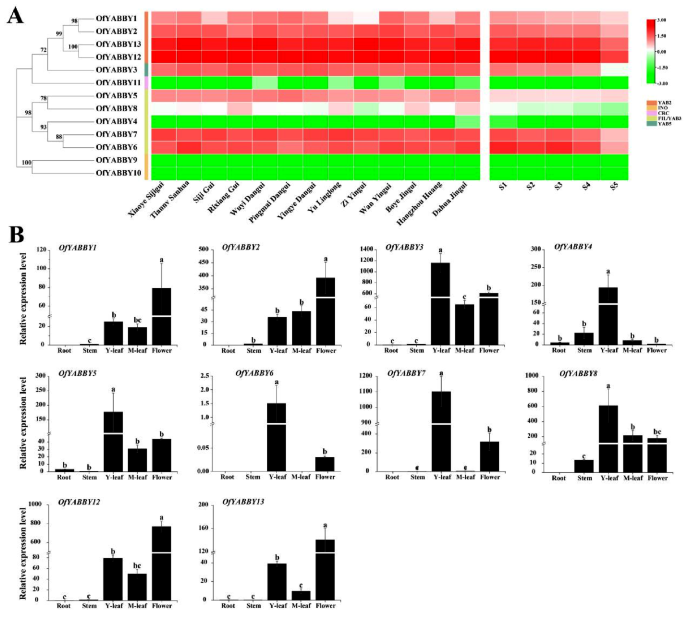
Transcriptome data were obtained to analyze the expression patterns of OfYABBYs in different O. fragrans cultivars [40] and different flowering stages (bud-pedicel stage, bud-eye stage, primary blooming stage, full blooming stage, and flower fading stage) (unpublished, Table S6). The results showed that two OfYABBY genes in the INO subfamily (OfYABBY9 and OfYABBY10) and OfYABBY11 in the CRC subfamily had limited expression or were not expressed in floral tissues. Previous studies have reported that INO and CRC subfamily genes are only expressed in reproductive organs, and it is hypothesized in this study that OfYABBYs in the CRC and INO subfamilies may not participate in the formation of floral organs. Unlike OfYABBY9, OfYABBY10, and OfYABBY11, OfYABBY genes in the FIL/YAB3, YAB2, and YAB5 subfamilies, except from OfYABBY4 and OfYABBY8, were highly expressed in floral tissues, especially OfYABBY12 and OfYABBY13 (Fig. 2A).
Expression analysis of OfYABBY genes. (A) Differential expression profiles of OfYABBY genes in 13 cultivars at the full blooming stage, and five flowering stages including the bud-pedicel stage (S1), bud-eye stage (S2), primary blooming stage (S3), full blooming stage (S4), and flower fading stage (S5) of Osmanthus fragrans ‘Rixiang Gui’ based on RNA sequencing data. (B) Expression patterns of OfYABBY genes were confirmed in roots, stems, leaves (young and mature leaves), and flowers (full blooming stage) using quantitative reverse-transcription polymerase chain reaction (qRT-PCR) analysis. Error bars indicate the standard deviations of three biological replicates. Different letters indicate a significant difference (p < 0.05) as determined by analysis of variance (ANOVA), which is based on Duncan’s multiple range test
This study further conducted qRT-PCR analysis of the expression levels of 10 OfYABBY genes from the FIL/YAB3, YAB2, and YAB5 subfamilies in the roots, stems, leaves (young and mature leaves), and floral tissues. The expression patterns of various OfYABBYs in different tissues showed significant differences (Fig. 2B). Overall, these 10 OfYABBY genes were slightly or not expressed in roots and stems. Among them, OfYABBY4/5/6/7/8 from the FIL/YAB3 subfamily exhibited preferential expression in young leaves, and OfYABBY3 from the YAB5 subfamily had low expression in roots, stems, and mature leaves, and highest expression in young leaves, followed by flowers. Notably, OfYABBY1/2/12/13 exhibited similar expression patterns, with significantly higher expression in flowers than in other tissues.
Subcellular localization and transcriptional activation activity of OfYABBYs
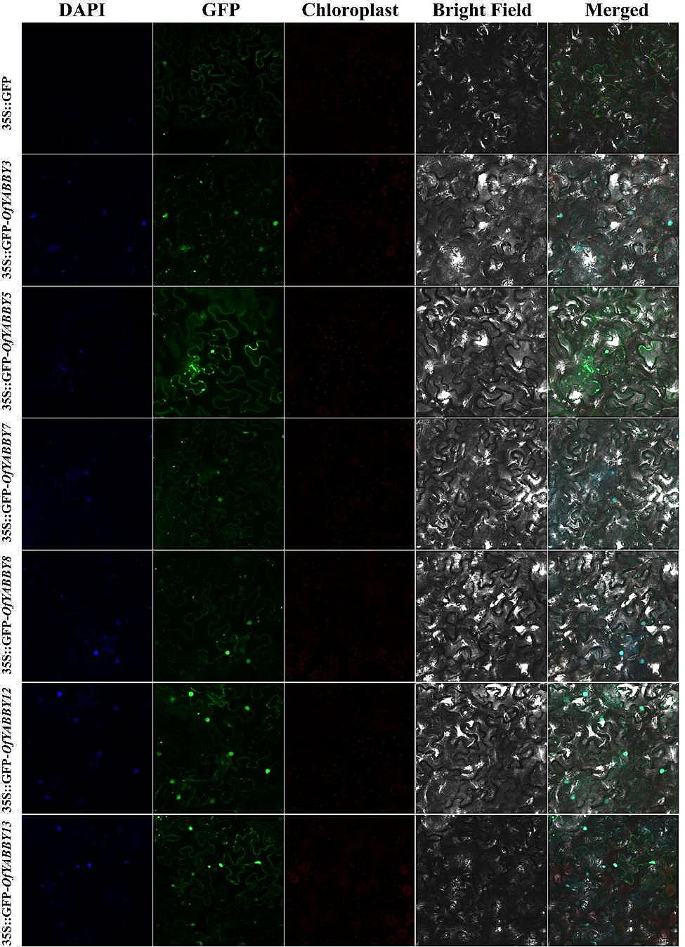
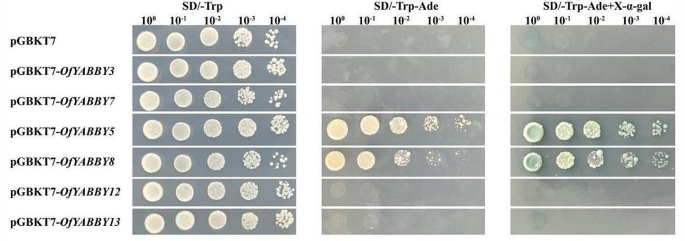
To examine the potential function of OfYABBY genes in transcriptional regulation, this study selected six OfYABBY genes from the FIL/YAB3, YAB2, and YAB5 subfamilies for subcellular localization analysis based on the bioinformatics analysis. Laser confocal microscopy revealed that the green fluorescent protein (GFP) fluorescence signals of 35 S::GFP-OfYABBY3/5/7/8/12/13 fusion proteins were detected mainly in the cell nuclei (Fig. 3). In addition, this work further cloned these six OfYABBY genes into the yeast expression vector pGBKT7 and found that yeast strains carrying the pGBKT7 empty vector (negative control) and the pGBKT7-OfYABBY vectors exhibited good growth on SD/-Trp culture medium. Only pGBKT7-OfYABBY5/8-transformed yeast could grow on SD/-Trp-Ade culture medium, and these strains showed normal growth and blue colonies on X-α-gal containing SD/-Trp-Ade culture medium (Fig. 4). This indicates that OfYABBY5/8 shows transcriptional activity in yeast, while OfYABBY3/7/12/13 does not show transcriptional activity.
Subcellular localization of selected OfYABBY proteins. The OfYABBY proteins fused with green fluorescent protein (GFP) were transiently expressed in tobacco leaf cells to observe subcellular localization through laser confocal microscopy. GFP fluorescence is shown in green and the nucleus is blue with 4’,6-diamidino-2-phenylindole (DAPI) staining
Phenotypes of transgenic N. tabacum overexpressing OfYABBY12
Plant YABBY transcription factors participate in the development of plant leaves and floral organs, as well as the biosynthesis of secondary metabolites, such as floral VOCs. Therefore, this study selected OfYABBY12, significantly more highly expressed in O. fragrans flowers than in other organs, for heterologous overexpression in tobacco. This allowed the further examination of the potential function of OfYABBYs. Positive transgenic tobacco plants were selected using semi-quantitative RT-PCR, and 10 transgenic lines were obtained (Fig. S4). Three lines with higher expression levels (lines 2, 5, and 12) were selected for subsequent analysis.
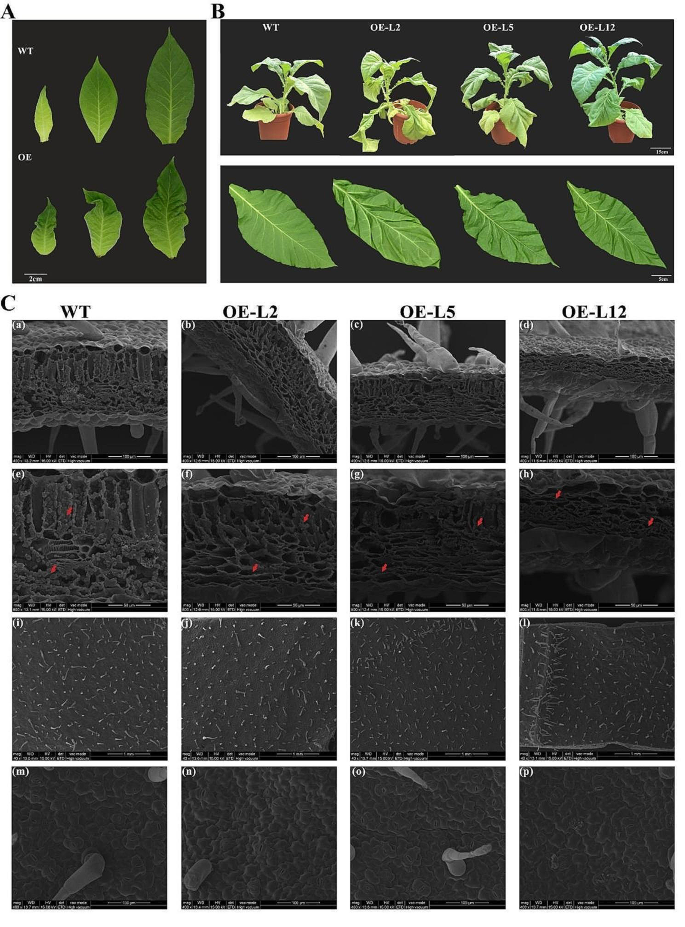
The phenotypic observation of tobacco leaves showed that, compared with the WT, the most intuitive phenotypic difference was that wrinkles appeared at the early stages of development in OfYABBY12-OE tobacco leaves and became more severe (Fig. 5A). Compared with WT tobacco, leaves in the overexpressing lines exhibited significant downward curvature and wrinkling (Fig. 5B), indicating that OfYABBY12 participated in the establishment of abaxial polarity in tobacco leaves. Scanning electron microscopy images of tobacco leaves (Fig. 5C) showed that the cross-sections of leaves from overexpression lines were significantly thinner, while the palisade and spongy mesophyll tissues were denser compared with WT tobacco (panels a–d). The leaf thickness of OfYABBY12-OE in lines 2, 5, and 12 decreased by 33, 33, and 57%, respectively, compared with the WT (Fig. S6). It was also observed that WT tobacco leaves were filled with granular inclusions, while there were significantly fewer inclusions in overexpressing lines (panels e–h). However, no significant difference was observed in leaf epidermal cells. Specifically, no significant differences were observed in epidermal hair (panels i–l) or stomata (panels m–p).
Phenotypic analysis of OfYABBY12 transgenic plants. (A) Leaf morphology of the wild-type (WT) and OfYABBY12-OE lines (overexpression of OfYABBY12 in tobacco (OE)) at different developmental stages. (B) OfYABBY12 transgenic plants produced curled leaves when compared with the WT. (C) Scanning electron microscopic images of tobacco leaves. (a–h) Transverse sections of tobacco leaves. (i–p) Epidermal structures of tobacco leaves. Red arrows show the inclusions in the tobacco leaves
Phenotypic observation of tobacco flowers showed that the pistil (style) of overexpressing tobacco lines was significantly extended compared with the WT, while the stamen length remained unchanged after OfYABBY12 gene expression was upregulated (Fig. S7).
Detection of VOCs in transgenic N. tabacum overexpressing OfYABBY12
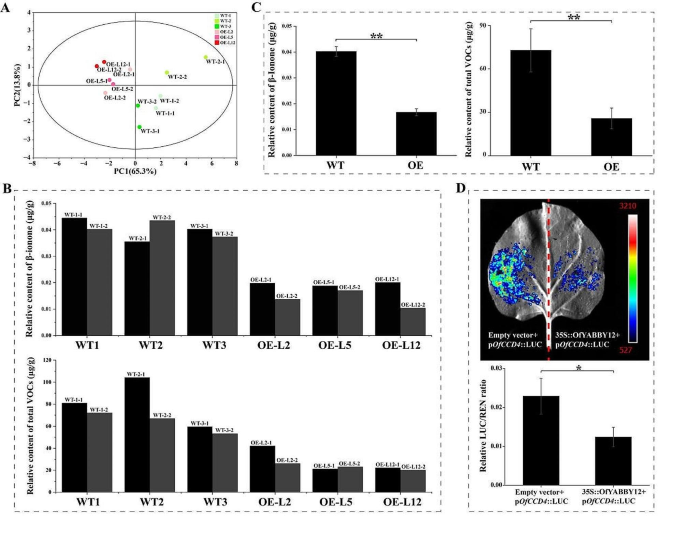
To further determine the function of OfYABBY12, this study assessed VOC levels in the leaves of OfYABBY12-OE tobacco plants. PCA revealed significant metabolic differences between WT and OfYABBY12-OE tobacco leaves (Fig. 6A), and variable importance in projection (VIP) values from OPLS-DA showed that β-ionone was the key differential metabolite between WT and OfYABBY12-OE tobacco leaves (Table S7). In OfYABBY12-OE tobacco leaves, the β-ionone content and total VOC content were both significantly lower than in the WT leaves (Figs. 6B and C). However, the GC–MS of the tobacco flowers showed no significant differences in VOCs released from WT and OfYABBY12-OE tobacco, and β-ionone was not detected in any of the tobacco flowers. PCA also showed that VOCs released by WT and overexpressing tobacco flowers could not be differentiated (Fig. S8).
Effects of OfYABBY12 overexpression on volatile organic compound (VOC) metabolism and the structure of tobacco leaves. (A) Principal component analysis (PCA) of VOCs in wild-type (WT) and OfYABBY12-overexpressing (OfYABBY12-OE) leaves. (B, C) Relative content analysis of β-ionone and total compounds of tobacco leaves. Two replicates per strain are shown in (B), and the means calculated from three strains with error bars reflecting standard deviations are shown in (C). (D) The dual-luciferase assay verified the relationship between OfYABBY12 and Pro-OfCCD4. Renilla (REN) luminescence was used to normalize the luciferase (LUC) activity. The mean ± standard deviation (SD) from three replicates is shown. Asterisks indicate significant differences (Student’s t-test: *P < 0.05; **P < 0.01)
In O. fragrans, carotenoid cleavage dioxygenase gene 4 (CCD4) positively regulates β-ionone biosynthesis [41, 42]. Thus, this study verified the regulatory relationship between OfYABBY12 and the OfCCD4 promoter using a dual-luciferase assay. Tobacco leaf cells had strong LUC signals, but the LUC/REN ratio of the experimental group (35 S::OfYABBY12 + pOfCCD4::LUC) was significantly lower than that of the control group (empty vector + pOfCCD4::LUC), indicating that OfYABBY12 negatively regulated the expression of OfCCD4 (Fig. 6D).
Subsequently, RNA-Seq analysis was conducted on tobacco leaves to study the effects of OfYABBY12 overexpression on the transcription levels in overexpressing tobacco. A total of 3721 differentially expressed genes (DEGs, expression change > 2-fold, p < 0.05) were identified (Fig. S9). The Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis of DEGs showed that these DEGs mainly participate in signaling pathways and metabolism in plants. Most DEGs found in the present study were concentrated in secondary metabolite synthetic pathways, such as in the biosynthesis of terpenes, flavonoids, and phenylpropanoids (Fig. 7A). In addition, Gene Ontology (GO) enrichment analysis of these DEGs found three GO entries in the top 20 enriched items related to terpene biosynthesis (Fig. 7B). Analysis of the DEGs that participate in terpene biosynthesis found that most upstream genes in the metabolic pathway were upregulated in the overexpressing lines. Among downstream genes, some NtTPSs were downregulated, and some were upregulated. NtCCD1 and NtCCD4 were significantly downregulated (Fig. 7C).
Transcriptome profiling of tobacco leaves. (A) Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis of differentially expressed genes (DEGs) in transgenic leaves. (B) Gene Ontology (GO) classification of unigenes among the annotated DEGs of OfYABBY12 overexpression in transgenic leaves. The bubble chart shows the enrichment of DEGs in certain pathways. (C) Overview of transcript changes in terpene biosynthesis pathway genes in wild-type (WT) and OfYABBY12-overexpressing (OfYABBY12-OE) leaves. DXS: 1-deoxy-D-xylulose-5-phosphate synthase; DXR: 1-deoxy-D-xylulose-5-phosphate reductoisomerase; MCT: 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase; CMK: 4-diphosphocytidyl-2-C-methyl-D-erythritol kinase; MDS: 2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase; HDS: (E)-4-hydroxy-3-methylbut-2-enyl-diphosphate synthase; HDR: 4-hydroxy-3-methylbut-2-enyl diphosphate reductase; AACT: acetyl-CoA C-acetyltransferase; HMGS: 3-hydroxy-3-methylglutaryl-CoA synthase; HMGR: 3-hydroxy-3-methylglutaryl CoA reductase; MVK: mevalonate kinase; PMK: phosphomevalonate kinase; MVD: diphosphomevalonate decarboxylase; TPS: terpene synthase; CCD: carotenoid cleavage dioxygenase
Discussion
Overview of the YABBY gene family in O. fragrans
The YABBY gene family has relatively low membership in different species and is a small gene family. For example, six, nine, and seven YABBY gene members have been identified in A. thaliana [39], S. lycopersicum [43], and V. vinifera [15], respectively. In this study, 13 OfYABBY genes were identified in the O. fragrans genome. This relatively high number may be the result of gene duplication events. This study did not find tandem repeats of the YABBY genes in O. fragrans. However, three segmental duplication YABBY genes were identified (Fig. 1C), indicating that the evolution of the O. fragrans YABBY gene family may have been driven by segmental duplication. In addition, genomic studies have shown that O. fragrans underwent two whole genome duplication events compared with A. thaliana and V. vinifera [30].
OfYABBYs in O. fragrans could be divided into five subfamilies based on phylogenetic and gene structural analysis. Genes in the same subfamily showed similar motifs and intron/exon structures, suggesting that they may have similar functions. In addition, an analysis of the promoter regions of O. fragrans YABBY genes showed that photo-responsive elements were present in all members and were the cis-acting elements with the highest quantity. These genes may play an important role in photo-response and photomorphogenesis, thereby affecting the growth and development of plant leaves. Moreover, some cis-acting elements involved in the developmental regulation of plant tissues and organs were identified. Previous studies have also reported that YABBY gene family members play extremely important roles in leaf development and synthesis [44,45,46,47]. In addition, the cis-acting elements include some plant hormone response elements, such as abscisic acid, auxin, and methyl jasmonate. One study reported that the interactions between A. thaliana FIL and JAZ proteins (an inhibitor of the jasmonic acid pathway) affected anthocyanidin accumulation [48]. O. fragrans OfYABBY1/2/4/6/7/8/9/10/11/12/13 all contain methyl jasmonate response elements. We speculate that these OfYABBY genes may be mediated by jasmonic acid signaling to regulate the synthesis of various secondary metabolites.
The function of O. fragrans YABBY genes can be predicted to some extent based phylogenetic relationships with previously studied YABBY genes. Consistent with previous studies, the O. fragrans YABBY genes were found to have closer phylogenetic relationships with YABBYs from dicotyledon plants than with those from monocotyledon plants (A. thaliana, S. lycopersicum, and V. vinifera) (Fig. 1B). As important model plants, the functions of the A. thaliana and S. lycopersicum YABBY genes have been extensively examined. These genes mainly play important roles in plant growth and development, particularly in leaf growth, fruit development, floral organ formation, and the synthesis of plant secondary metabolites [14, 27, 49, 50]. In addition, subcellular localization analysis showed that 35 S::GFP-OfYABBY3/5/7/8/12/13 fusion proteins were mainly located in the nucleus, suggesting that these proteins have diverse functions in the nucleus. Transcriptional activity analysis revealed that OfYABBY5/8 exhibited transcriptional activity in yeast cells and may directly regulate downstream target genes. However, OfYABBY3/7/12/13 did not exhibit transcriptional activity in yeast cells, and thus, these proteins may require interactions with other transcription factors or specific environmental conditions to perform their regulatory functions.
OfYABBY12 plays a negative role in VOC synthesis
YABBY transcription factors have been reported to have tissue specificity in plants and to play different roles in different plant tissues [44]. In the present study, OfYABBY9, OfYABBY10, and OfYABBY11 were not detected or exhibited extremely low transcriptional expression in different cultivars or different flowering stages in O. fragrans floral organs. In A. thaliana, AtINO and AtCRC are only expressed in reproductive organs [22, 23]; OfYABBY9, OfYABBY10, and AtINO are in the same subfamily, and OfYABBY11 and AtCRC in the same subfamily (Fig. 1B). This suggests that O. fragrans CRC and INO subfamily genes may not participate in regulating the development of floral organs. However, the FIL/YAB3, YAB2, and YAB5 subfamily genes, including OfYABBY1/2/3/6/7/12/13, are highly expressed in O. fragrans leaves or flowers but lowly expressed or not expressed in roots and stems. This suggests that these genes may play important roles in O. fragrans leaves and floral organs. Furthermore, studies have reported that there is redundancy in the functions of genes in the FIL/YAB3, YAB2, and YAB5 subfamilies [22, 51], and these genes often participate in regulating leaf morphogenesis, the development and differentiation of lateral organs, and the biosynthesis of secondary metabolites [8, 11, 13, 17, 28].
Notably, comparing the transcript levels of all OfYABBYs revealed that OfYABBY12 in the YAB2 subfamily had the highest transcript level (Fig. 2A) and showed significant differences in expression levels among O. fragrans roots, stems, young leaves, mature leaves, and floral organs (Fig. 2B). Therefore, this study selected OfYABBY12 for further functional validation. However, a stable genetic transformation system for O. fragrans has not yet been established. Thus, OfYABBY12 was stably overexpressed in tobacco to determine whether OfYABBY12 regulates the differentiation and formation of tobacco leaves and floral organs, as well as whether it affects their volatile metabolites. In the present study, RNA-Seq analysis of WT and OfYABBY12-OE tobacco leaves found that most DEGs were enriched in metabolic pathways related to the synthesis of terpenes, flavonoids, and phenylpropanoids (Figs. 7A and B). GC–MS results showed that there were significant differences in the VOCs between WT and overexpressing tobacco leaves. The content of β-ionone and total VOCs were significantly decreased (Figs. 6A and C), and critical genes that participate in β-ionone synthesis, such as NtCCD1 and NtCCD4, were significantly downregulated in overexpressing tobacco leaves (Fig. 7C). It has also been reported that MsYABBY5 negatively regulates the synthesis of volatile terpene compounds in M. spicata [8]. β-Ionone is a critical compound for floral scent synthesis in O. fragrans [52]. Our previous study also found that OfYABBY12 in the molecular regulatory network of β-ionone synthesis showed a significant negative correlation with critical enzyme genes in its metabolic pathway (Fig. S10) [40]. Correspondingly, the dual-luciferase assay in the present study showed that OfYABBY12 negatively regulated the expression of OfCCD4, which has been reported to promote β-ionone synthesis [41, 42]. The results suggest that OfYABBY12 negatively regulates β-ionone synthesis in O. fragrans.
In addition, it was observed that the inclusions of OfYABBY12-OE tobacco leaves were significantly decreased compared with the WT under scanning electron microscopy (Fig. 5C), but whether this is related to the reduction of VOCs needs further study. However, OfYABBY12 overexpression did not significantly affect VOCs from tobacco flowers. This suggests that OfYABBY12 overexpression affects VOCs from tobacco leaves, but not from tobacco flowers. Further studies are also needed to determine the regulatory relationship.
OfYABBY12 is involved in the development of leaves and flowers
In the present study, the overexpression of OfYABBY12 in tobacco revealed that OfYABBY12 overexpression affected the formation of the adaxial–abaxial axis in tobacco leaves, causing leaves to curl and crenations to occur. In Saccharum spontaneum, SsYABBY2 overexpression causes the abaxial side of A. thaliana leaves to curl [53]. Overexpression of C. sinensis CsFIL and CsYAB2 in A. thaliana causes leaves to curl [33]. Furthermore, one study found that the overexpression of the V. vinifera VvYABBY4 gene in S. lycopersicum caused the pistils (stigma) of transgenic S. lycopersicum to become longer [15], while the downregulation of MlYAB1, MlYAB2, MlYAB3, and MlYAB5 in M. lewisii inhibited style elongation [34]. In the present study, OfYABBY12, VvYABBY4, and MlYAB2 were in the same subfamily (Figs. 1B and S3). Significant elongation was also observed in the pistils (stigma) of OfYABBY12-OE tobacco flowers, while anther length remained unchanged.
Conclusion
The present study identified 13 OfYABBY genes in the O. fragrans genome, which were classified into five subfamilies. Phylogenetic analysis and gene duplication events demonstrated that gene duplication aided in the expansion of the O. fragrans OfYABBY gene family and that the gene functions of the OfYABBY family may be conserved. The genes in the YAB2, FIL/YAB3, and YAB5 subfamilies may have overlapping functions. In addition, the OfYABBY gene expression pattern analysis indicated that OfYABBY genes in the YAB2, FIL/YAB3, and YAB5 subfamilies may play important roles in O. fragrans leaf and/or floral organs. Functional validation showed that OfYABBY12 significantly affected the VOCs from tobacco leaves, especially β-ionone, and the dual-luciferase assay revealed that OfYABBY12 negatively regulated the expression of OfCCD4, which promoted β-ionone synthesis. OfYABBY12 also played an important role in the establishment of polarity in tobacco leaves and in the development of lateral organs (pistils). The above results suggest that the OfYABBY gene family may participate in the growth and development of O. fragrans leaves and lateral organs, as well as in the synthesis of O. fragrans floral scent. In conclusion, this study provides a foundation for further research on the YABBY gene family in O. fragrans as well as new findings regarding the biosynthesis of the floral scent substance β-ionone in O. fragrans.
Data availability
All data generated or analyzed during this study are included in this published article, its supplementary information files and publicly available repositories. RNA-Seq raw data were uploaded to the NCBI sequence read archive (http://www.ncbi.nlm.nih.gov/sra/) under accession number SRP450701 and are accessible under Bioproject archive number PRJNA997126 (http://www.ncbi.nlm.nih.gov/bioproject/).
References
Wang LM, Li MT, Jin WW, Li S, Zhang SQ, Yu LJ. Variations in the components of Osmanthus fragrans Lour. Essential oil at different stages of flowering. Food Chem. 2009;114:233–6.
Zhou F, Zhao YJ, Li MQ, Xu T, Zhang LQ, Lu BY, Wu XD, Ge ZW. Degradation of phenylethanoid glycosides in Osmanthus fragrans Lour. Flowers and its effect on anti-hypoxia activity. Sci Rep. 2017;7:10068.
Han Y, Dong MF, Yuan WJ, Shang FD. Study on the genetic diversity of Osmanthus fragrans cultivars. Chin Bull Bot. 2008;25:559–64.
Chen HG, Zeng XL, Yang J, Cai X, Shi YM, Zheng RR, Wang ZQ, Liu JY, Yi XX, Xiao SW, Fu Q, Zou JJ, Wang CY. Whole-genome resequencing of Osmanthus fragrans provides insights into flower color evolution. Hortic Res. 2021;8:98.
Feng YY, Li QY, Huang JH, Hu SQ. Numerical classification of 25 color-leafed Osmanthus fragrans clones (cultivars). J Nanjing Forestry Univ (Natural Sci Edition). 2021;45:107–15.
Stahle MI, Kuehlich J, Staron L, von Arnim AG, Golz JF. YABBYs and the transcriptional corepressors LEUNIG and LEUNIG_HOMOLOG maintain leaf polarity and meristem activity in Arabidopsis. Plant Cell. 2009;21:3105–18.
Sarojam R, Sappl PG, Goldshmidt A, Efroni I, Floyd SK, Eshed Y, Bowman JL. Differentiating Arabidopsis shoots from leaves by combined YABBY activities. Plant Cell. 2010;22:2113–30.
Wang Q, Reddy VA, Panicker D, Mao HZ, Kumar N, Rajan C, Venkatesh PN, Chua NH, Sarojam R. Metabolic engineering of terpene biosynthesis in plants using a trichome-specific transcription factor MsYABBY5 from spearmint (Mentha spicata). Plant Biotechnol J. 2016;14:1619–32.
Zhang T, Li C, Li D, Liu Y, Yang X. Roles of YABBY transcription factors in the modulation of morphogenesis, development, and phytohormone and stress responses in plants. J Plant Res. 2020;133:751–63.
Chen YY, Hsiao YY, Chang SB, Zhang D, Lan SR, Liu ZJ, Tsai WC. Genome-wide identification of YABBY genes in Orchidaceae and their expression patterns in Phalaenopsis orchid. Genes. 2020;11:955.
Eckardt NA. YABBY genes and the development and origin of seed plant leaves. Plant Cell. 2010; 22: 2103.
Satterlee JW, Scanlon MJ. Coordination of leaf development across developmental axes. Plants. 2019;8:433.
Zhang XL, Zhang LG. Molecular cloning and expression of the male sterility-related CtYABBY1 gene in flowering Chinese cabbage (Brassica campestris L. Ssp chinensis var. Parachinensis). Genet Mol Res. 2014;13:4336–47.
Strable J, Vollbrecht E. Maize YABBY genes drooping leaf1 and drooping leaf2 regulate floret development and floral meristem determinacy. Development. 2019;146:dev171181.
Zhang S, Wang L, Sun X, Li Y, Yao J, Nocker SV, Wang X. Genome-wide analysis of the YABBY gene family in grapevine and functional characterization of VvYABBY4. Front Plant Sci. 2019;10:1207.
Jie GUO, Zhou XT, Dai KL, Yuan XY, Guo PY, Shi WP, Zhou MX. Comprehensive analysis of YABBY gene family in foxtail millet (Setaria italica) and functional characterization of SiDL. J Integr Agr. 2022;21:2876–87.
Kayani SI, Shen Q, Ma YN, Fu XQ, Xie LH, Zhong YJ, Tiantian C, Pan QF, Li L, Rahman US, Sun XF, Tang KX. The YABBY family transcription factor AaYABBY5 directly targets cytochrome P450 monooxygenase (CYP71AV1) and double-bond reductase 2 (DBR2) involved in artemisinin biosynthesis in Artemisia Annua. Front Plant Sci. 2019;10:1084.
Kayani SI, Shen Q, Rahman SU, Fu XQ, Li YP, Wang C, Hassani D, Tang KX. Transcriptional regulation of flavonoid biosynthesis in Artemisia annua by AaYABBY5. Hortic Res. 2021;8:257.
Zhao SP, Lu D, Yu TF, Ji YJ, Zheng WJ, Zhang SX, Chai SC, Chen ZY, Cui XY. Genome-wide analysis of the YABBY family in soybean and functional identification of GmYABBY10 involvement in high salt and drought stresses. Plant Physiol Bioch. 2017;119:132–46.
Yang ZE, Gong Q, Wang LL, Jin YY, Xi JP, Li Z, Qin WQ, Yang ZR, Lu LL, Chen QJ, Li FG. Genome-wide study of YABBY genes in upland cotton and their expression patterns under different stresses. Front Genet. 2018;9:33.
Li ZY, Li G, Cai MX, Priyadarshani SV, Aslam M, Zhou Q, Huang XY, Wang XM, Liu YQ, Qin Y. Genome-wide analysis of the YABBY transcription factor family in pineapple and functional identification of AcYABBY4 involvement in salt stress. Int J Mol Sci. 2019;20:5863.
Bowman JL, Smyth DR. CRABS CLAW, a gene that regulates carpel and nectary development in Arabidopsis, encodes a novel protein with zinc finger and helix-loop-helix domains. Development. 1999;126:2387–96.
Villanueva JM, Broadhvest J, Hauser BA, Meister RJ, Schneitz K, Gasser CS. INNER NO OUTER regulates abaxial-adaxial patterning in Arabidopsis ovules. Gene Dev. 1999;13:3160–9.
Sun L, Wei YQ, Wu KH, Yan JY, Xu JN, Wu YR, Li GX, Xu JM, Harberd NP, Ding ZJ, Zheng SJ. Restriction of iron loading into developing seeds by a YABBY transcription factor safeguards successful reproduction in Arabidopsis. Mol Plant. 2021;14:1624–39.
Filyushin MA, Slugin MA, Dzhos EA, Kochieva EZ, Shchennikova AV. Coexpression of YABBY1 and YABBY3 genes in lateral organs of tomato species (Solanum, Section Lycopersicon). Dokl Biochem Biophys. 2018;478:50–4.
Bartley GE, Ishida BK. Ethylene-sensitive and insensitive regulation of transcription factor expression during in vitro tomato sepal ripening. J Exp Bot. 2007;58:2043–51.
Yang T, He Y, Niu S, Zhang Y. A YABBY gene CRABS CLAW a (CRCa) negatively regulates flower and fruit sizes in tomato. Plant Sci. 2022;320:111285.
Zhang X, Ding L, Song AP, Li S, Liu JY, Zhao WQ, Jia DW, Guan YX, Zhao KK, Chen SM, Jiang JF, Chen FD. DWARF AND ROBUST PLANT regulates plant height via modulating gibberellin biosynthesis in chrysanthemum. Plant Physiol. 2022;190:2484–500.
Bonaccorso O, Lee JE, Puah L, Scutt CP, Golz JF. FILAMENTOUS FLOWER controls lateral organ development by acting as both an activator and a repressor. BMC Plant Biol. 2012;12:1–16.
Yang XL, Yue YZ, Li HY, Ding WJ, Chen GW, Shi TT, Chen JH, Park MS, Chen F, Wang LG. The chromosome-level quality genome provides insights into the evolution of the biosynthesis genes for aroma compounds of Osmanthus fragrans. Hortic Res. 2018;5:72.
Finn RD, Coggill PY, Eberhardt RY, Eddy SR, Mistry J, Mitchell AL, Potter SC, Punta M, Qureshi M, Sangrador-Vegas A, Salazar GA, Tate J, Bateman A. The pfam protein families database: towards a more sustainable future. Nucleic Acids Res. 2016;44:D279–85.
Chen CJ, Chen H, Zhang Y, Thomas HR, Frank MH, He YH, Xia R. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020;13:1194–202.
Shen Y, Li XM, Ma GL, Zhao Y, Jiang XL, Gao LP, Xia T, Liu YJ. Roles of YABBY tanscription factors in the regulation of leaf development and abiotic stress responses in Camellia sinensis. Bev Plant Res. 2022;2:1–10.
Ding BQ, Li JJ, Gurung VD, Lin QS, Sun XM, Yuan YW. The leaf polarity factors SGS3 and YABBYs regulate style elongation through auxin signaling in Mimulus lewisii. New Phytol. 2021;232:2191–206.
Li ZN, Jiang YJ, Liu DF, Ma J, Li J, Li MY, Sui SZ. Floral scent emission from nectaries in the adaxial side of the innermost and middle petals in Chimonanthus praecox. Int J Mol Sci. 2018;19:3278.
Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–9.
Zhang C, Fu JX, Wang YG, Bao ZY, Zhao HB. Identification of suitable reference genes for gene expression normalization in the quantitative real-time PCR analysis of sweet osmanthus (Osmanthus fragrans Lour). PLoS ONE. 2015;10:e0136355.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2. Method Methods. 2001;25:402–8.
Siegfried KR, Eshed Y, Baum SF, Otsuga D, Drews GN, Bowman JL. Members of the YABBY gene family specify abaxial cell fate in Arabidopsis. Development. 1999;126:4117–28.
Yue YZ, Shi TT, Liu JW, Tian QY, Yang XL, Wang LG. Genomic, metabonomic and transcriptomic analyses of sweet osmanthus varieties provide insights into floral aroma formation. Sci Hortic. 2022;306:111442.
Huang FC, Molnar P, Schwab W. Cloning and functional characterization of carotenoid cleavage dioxygenase 4 genes. J Exp Bot. 2009;60:3011–22.
Zhang XS, Pei JS, Zhao LG, Tang F, Fang XY, Xie JC. Overexpression and characterization of CCD4 from Osmanthus fragrans and β-ionone biosynthesis from β-carotene in vitro. J Mol Catal B-Enzym. 2016;134:105–14.
Han HQ, Liu Y, Jiang MM, Ge HY, Chen HY. Identification and expression analysis of YABBY family genes associated with fruit shape in tomato (Solanum lycopersicum L). Genet Mol Res. 2015;14:7079–91.
Bowman JL. The YABBY gene family and abaxial cell fate. Curr Opin Plant Biol. 2000;3:17–22.
Wang AJ, Tang JF, Li DY, Chen CY, Zhao XY, Zhu LH. Isolation and functional analysis of LiYAB1, a YABBY family gene, from lily (Lilium longiflorum). J Plant Physiol. 2009;166:988–95.
Du F, Guan C, Jiao Y. Molecular mechanisms of leaf morphogenesis. Mol Plant. 2018;11:1117–34.
Wang HF, Kong FJ, Zhou CE. From genes to networks: the genetic control of leaf development. J Integr Plant Biol. 2021;63:1181–96.
Boter M, Golz JF, Giménez-Ibañez S, Fernandez-Barbero G, Franco-Zorrilla JM, Solano R. FILAMENTOUS FLOWER is a direct target of JAZ3 and modulates responses to jasmonate. Plant Cell. 2015;27:3160–74.
Kumaran MK, Bowman JL, Sundaresan V. YABBY polarity genes mediate the repression of KNOX homeobox genes in Arabidopsis. Plant Cell. 2002;14:2761–70.
Garrido-Bigotes A, Torrejón M, Solano R, Figueroa CR. Interactions of JAZ repressors with anthocyanin biosynthesis-related transcription factors of Fragaria×ananassa. Agronomy. 2020;10:1586.
Goldshmidt A, Alvarez JP, Bowman JL, Eshed Y. Signals derived from YABBY gene activities in organ primordia regulate growth and partitioning of Arabidopsis shoot apical meristems. Plant Cell. 2008;20:1217–30.
Han YJ, Wang HY, Wang XD, Li K, Dong MF, Li Y, Zhu Q, Shang FD. Mechanism of floral scent production in Osmanthus fragrans and the production and regulation of its key floral constituents, β-ionone and linalool. Hortic Res. 2019;6:106.
She ZY, Huang XY, Aslam M, Wang LL, Yan MK, Qin RJ, Chen YZ, Qin Y, Niu XP. Expression characterization and cross-species complementation uncover the functional conservation of YABBY genes for leaf abaxial polarity and carpel polarity establishment in Saccharum spontaneum. BMC Plant Biol. 2022;22:124.
Acknowledgements
We would like to thank all of the colleagues in our laboratory for providing useful discussions and technical assistance.
Funding
This work was supported by the National Natural Science Foundation of China (32071828) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Contributions
T.T.S. and Y.Z.Y. conceived and designed the research; T.T.S. and Y.F.Y. performed all experiments; X.L.Y. and L.G.W. provided technical assistance; T.T.S. wrote the manuscript; and L. Z., Y.Z.Y., and L.G.W. revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shi, T., Zhou, L., Ye, Y. et al. Characterization of YABBY transcription factors in Osmanthus fragrans and functional analysis of OfYABBY12 in floral scent formation and leaf morphology. BMC Plant Biol 24, 589 (2024). https://doi.org/10.1186/s12870-024-05047-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-024-05047-y